The mobile phone in your pocket is powered by a small, light-weight, powerful lithium-ion battery containing natural minerals that are extracted, or mined. Follow the global journey for five of these minerals to see the critical role they play in the development of the battery.
Resource Extraction: Where the supply chain begins.
Many materials for many functions
Lithium-ion batteries can have a variety of physical and chemical compositions based on a device’s needs for battery size, power, and capacity. Lithium is just one of the many chemical elements that can be found in a lithium-ion battery.
Student Reading: Lithium-ion Batteries by the Numbers (PDF 11mbs)
Two types of mining required to extract minerals for batteries are open-pit mining and brine extraction.
Student Reading: The Green Scene (PDF 23mbs)
Open-Pit Mining
Open-pit mining uses a descending set of ledges dug into the ground. Borers drill holes into the rock. Explosives are inserted into these holes to break the rock into boulders, which are then hauled away to be processed.
Brine Extraction
Brine extraction is a method in which miners pump groundwater to the surface that collects in ponds. Here, they wait for the water to evaporate under the sun’s heat. Left behind is a sludge residue, which contains the desired mineral.
Let’s explore a few of the resources that are in your phone’s battery: cobalt, copper, lithium, aluminum, and carbon in the form of graphite.
Cobalt
Co

Cobalt is a chemical element often added to alloys, two or more metallic elements, to improve their strength at high temperatures.
Cobalt is used as part of an active material (LiCoO2) that is applied to the cathode and acts as a lithium receptor in the electrochemical charge-discharge process.
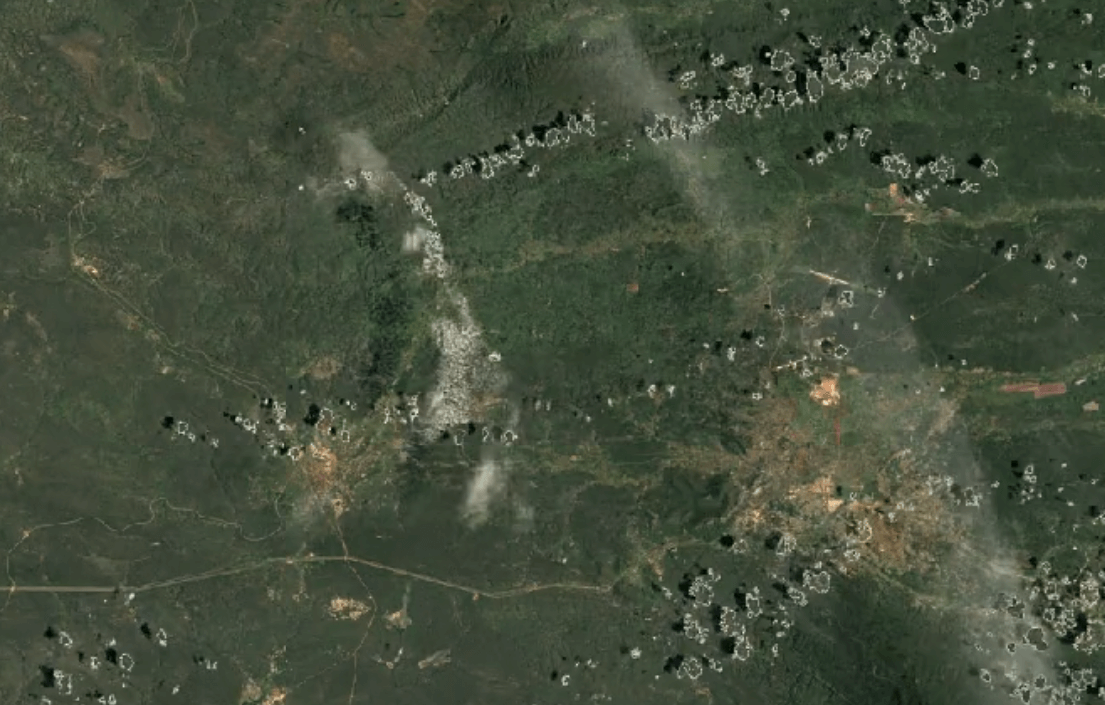
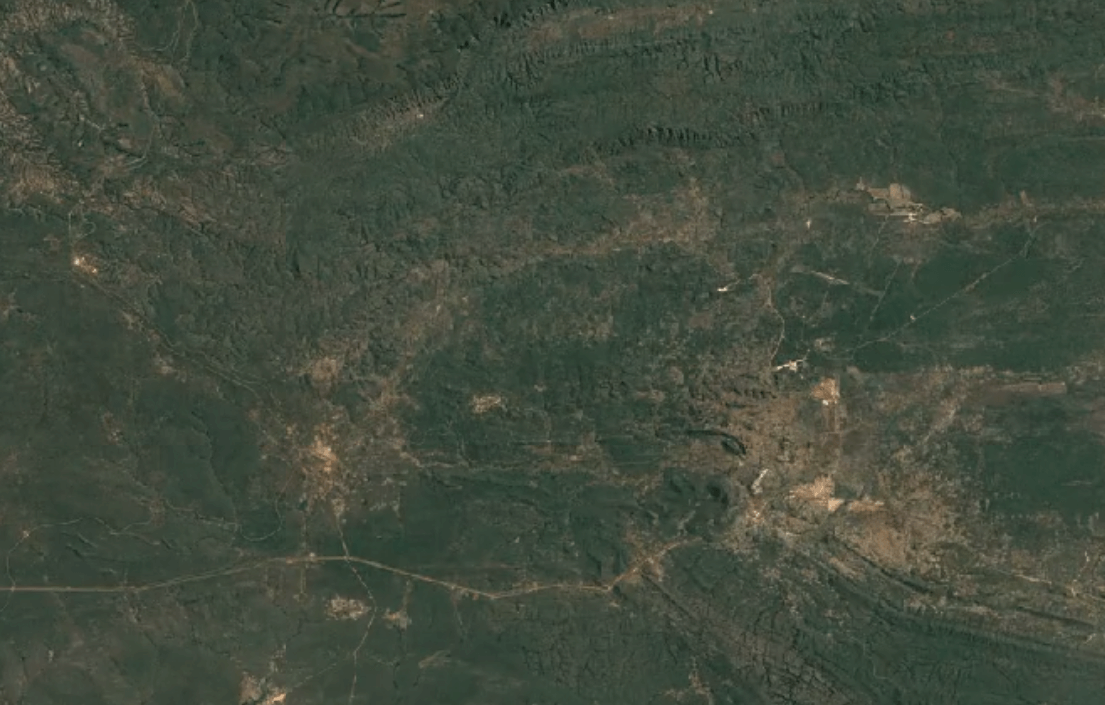
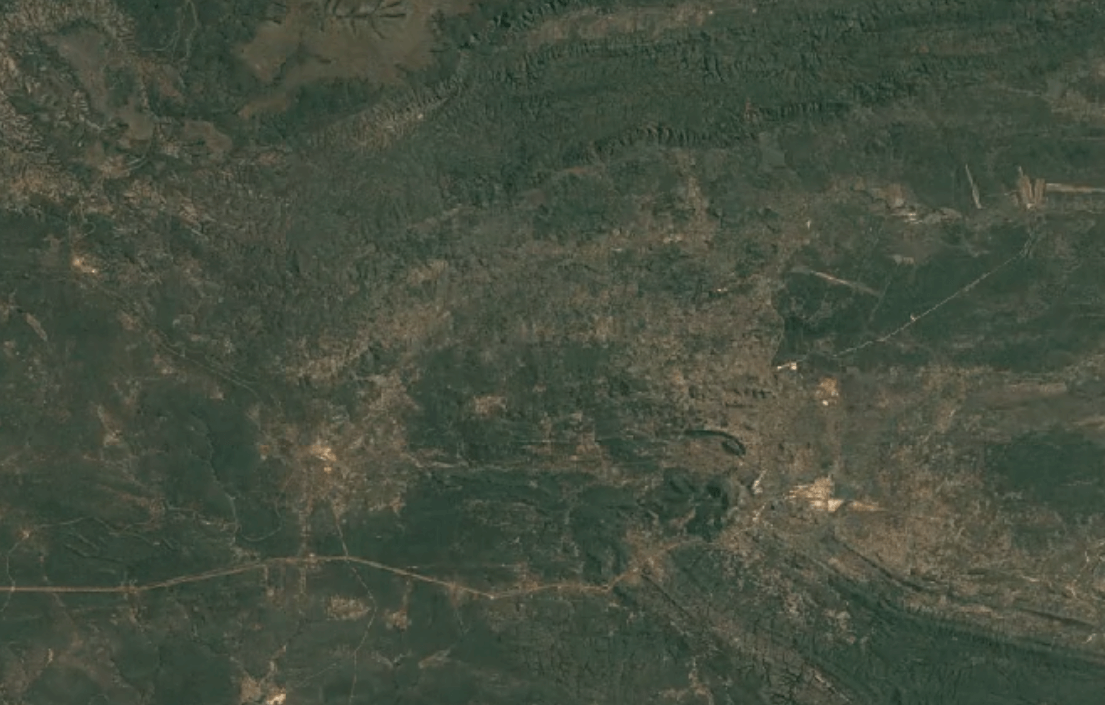
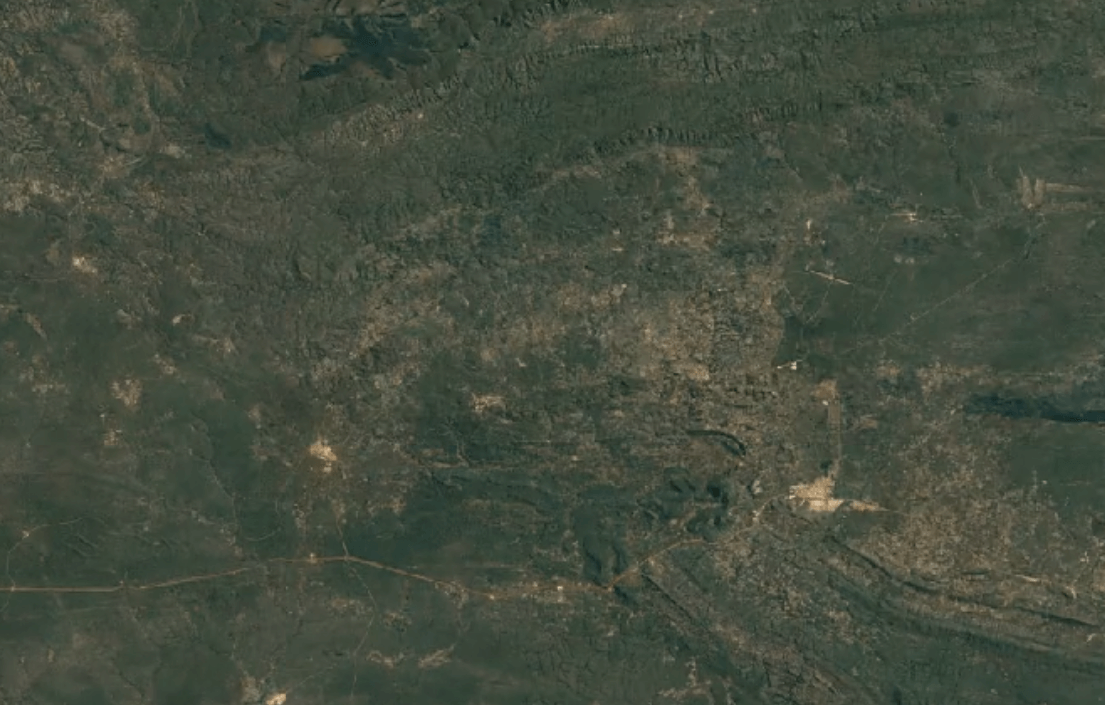
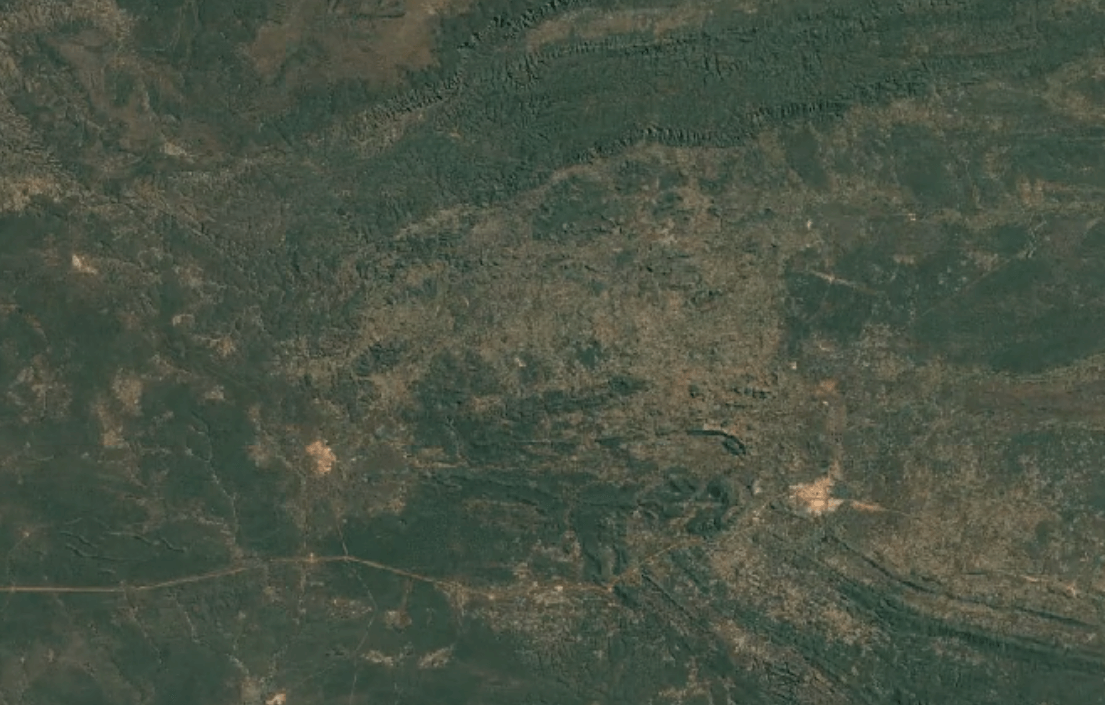
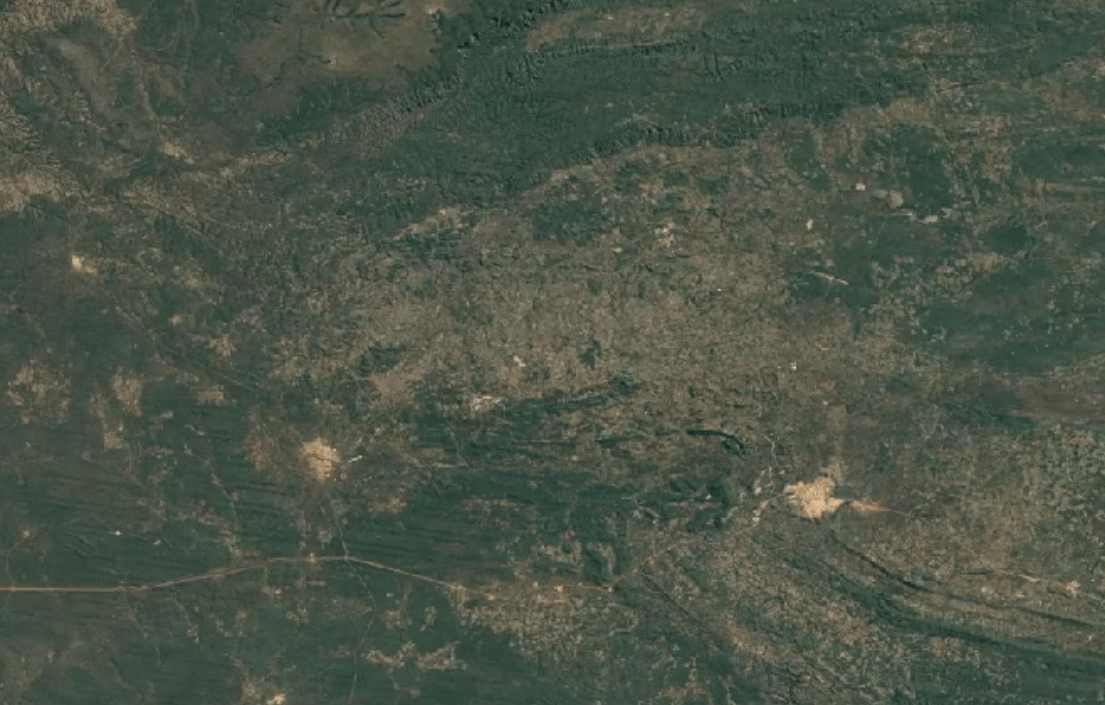
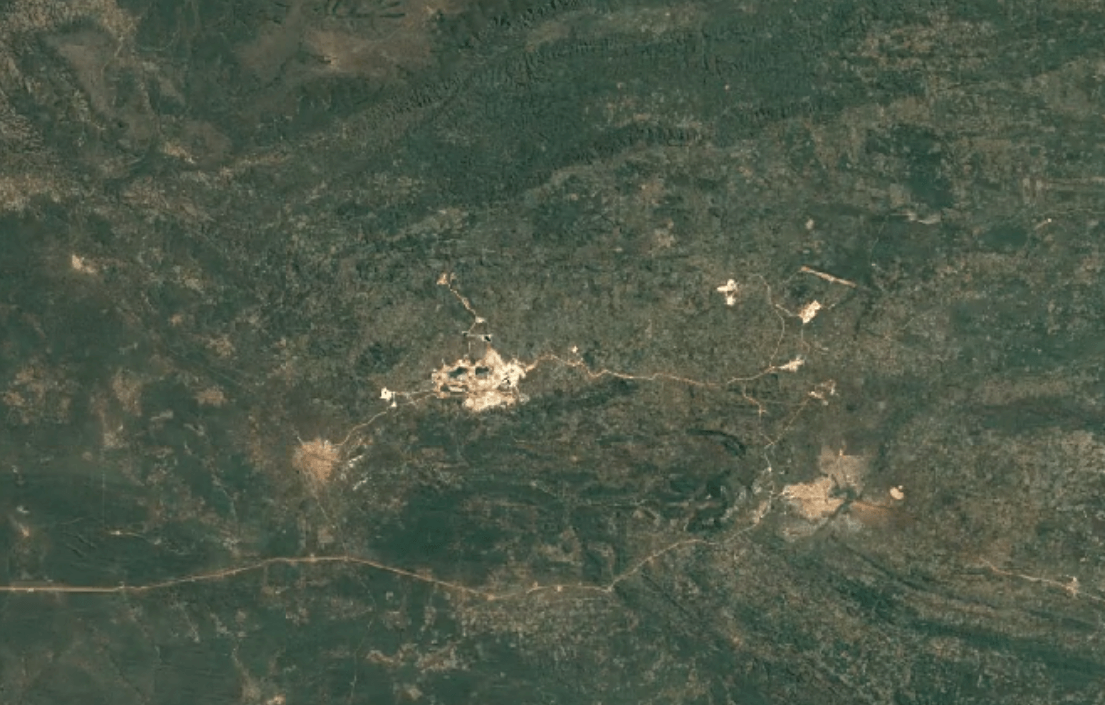
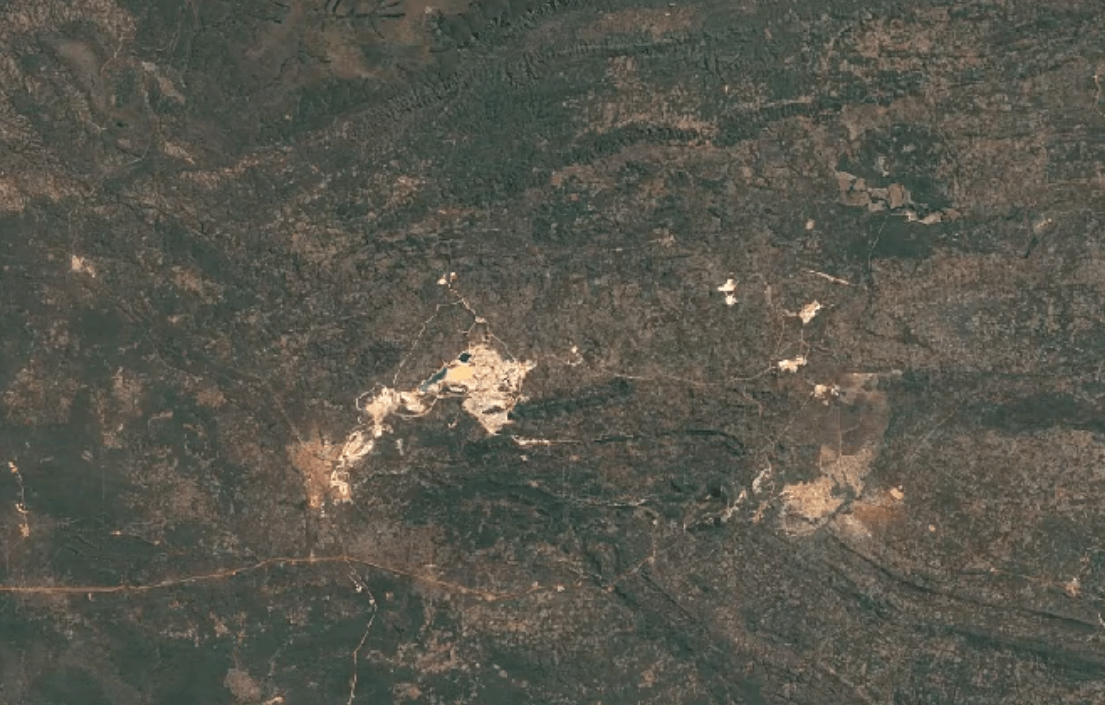
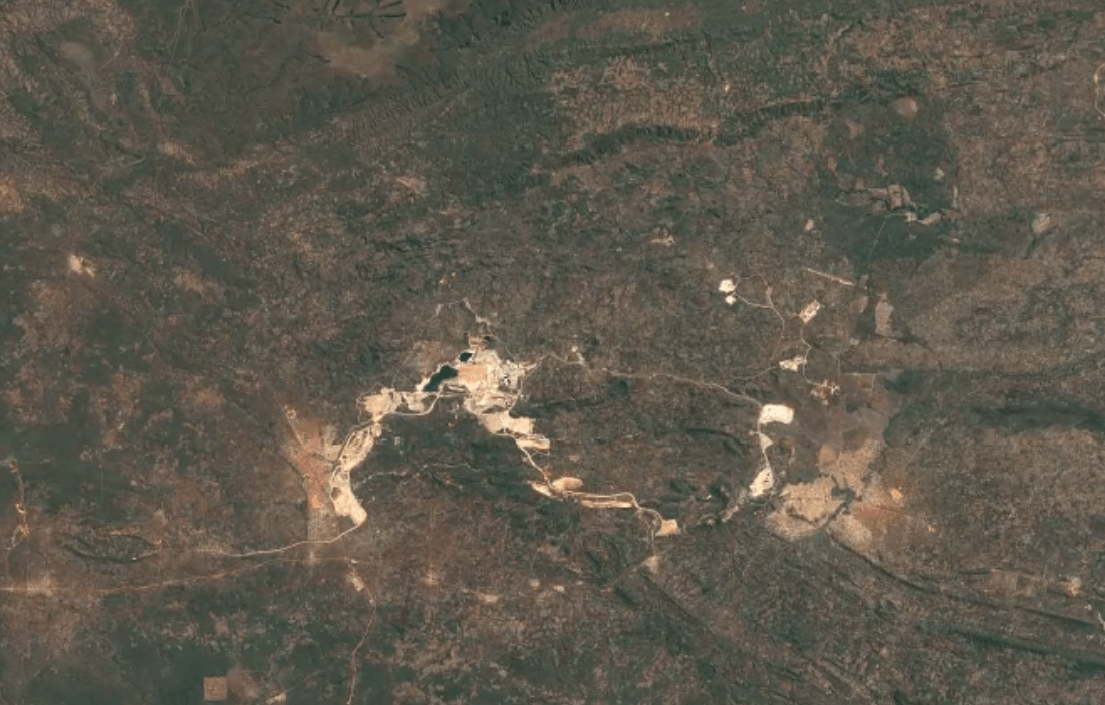
-
Tenke Fungurume, DR CongoImage Attribution: Google Earth Engine
Cobalt is usually mined in open-pit mines as a byproduct of mining other ores, especially nickel and copper – often, traces of cobalt can be found in these materials.
A substantial portion of the world’s cobalt is mined in unofficial, “clandestine” mines in which adults and children dig tunnels by hand deep underground. This is extremely unsafe: toxic fumes are in high concentrations, and collapsed tunnels are common.
Human and environmental health risks from mining:
Open-pit mining impacts the environment, and impacts people too. First, in order to make way for an open pit, vegetation must be cleared away. Then, a deep pit is dug. Together, these factors create conditions for erosion.
Mining can create toxic soils and dust with high concentrations of heavy metals like copper and nickel. These dusts are carried by wind into water supplies, crops, and the air. Breathing in, eating food, or drinking water with this contamination puts people and animals at a higher risk of illness.
Copper
Cu
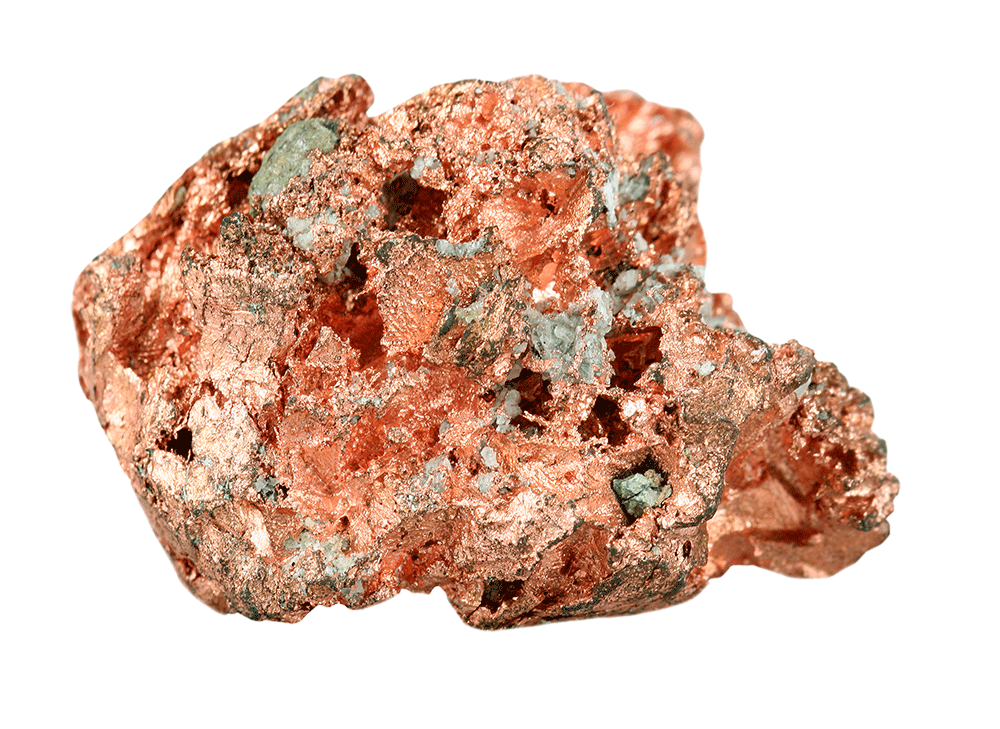
Refined copper is a very ductile metal: it can be easily shaped into a thin foil, wire, or thread. Copper is also highly conductive, thermally and electrically.
Because of these qualities, copper foil is used as the current collector at the anode of a lithium-ion battery. In the lithium-ion battery of a mobile phone, current collectors take the form of a foil and must be conductive enough to receive the electrical current.





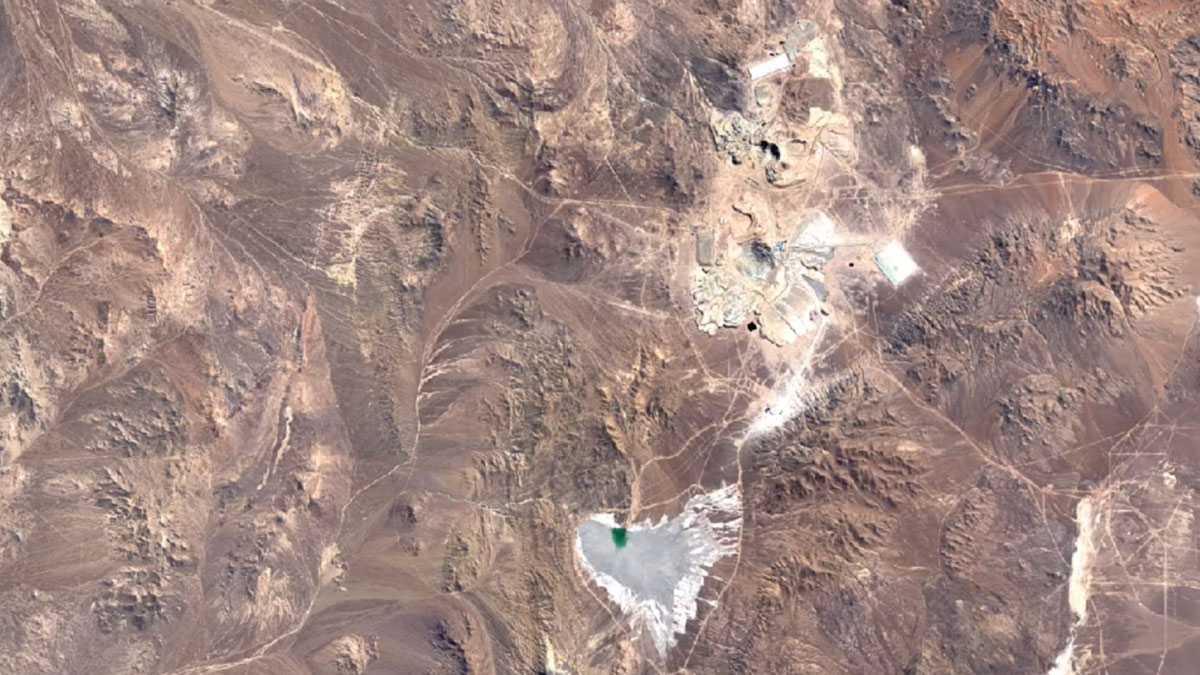
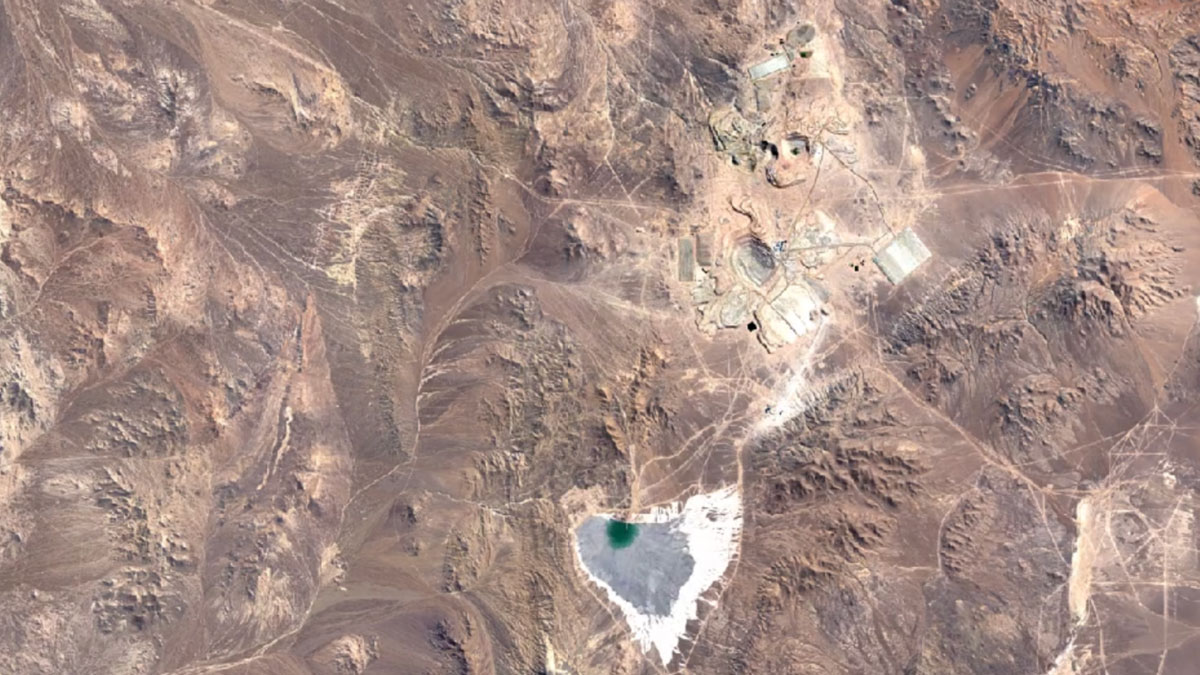


Escondida, ChileImage Attribution: Google Earth Engine
Most copper is mined using open-pit mining.
Human and environmental health risks from mining:
Open-pit mining impacts the environment, and impacts people too. First, in order to make way for an open pit, vegetation must be cleared away. Then, a deep pit is dug. Together, these factors create conditions for erosion.
Mining can create toxic soils and dust with high concentrations of heavy metals like copper and nickel. These dusts are carried by wind into water supplies, crops, and the air. Breathing in, eating food, or drinking water with this contamination puts people and animals at a higher risk of illness.
Lithium
Li

Lithium is the lightest solid element in the Periodic Table.
- Lithium in the form of lithium-ion (Li+) moves between electrodes. The movement of Li+ is responsible for creating an electrical current.
- Lithium in the form of an oxide (LiCoO2, or lithium cobalt oxide) is used as the active component of the cathode.
Li+ ions move between the battery’s cathode and anode internally, and electrons move in the opposite direction in the external circuit. This migration is the reason the battery powers the device, because it creates the electrical current.




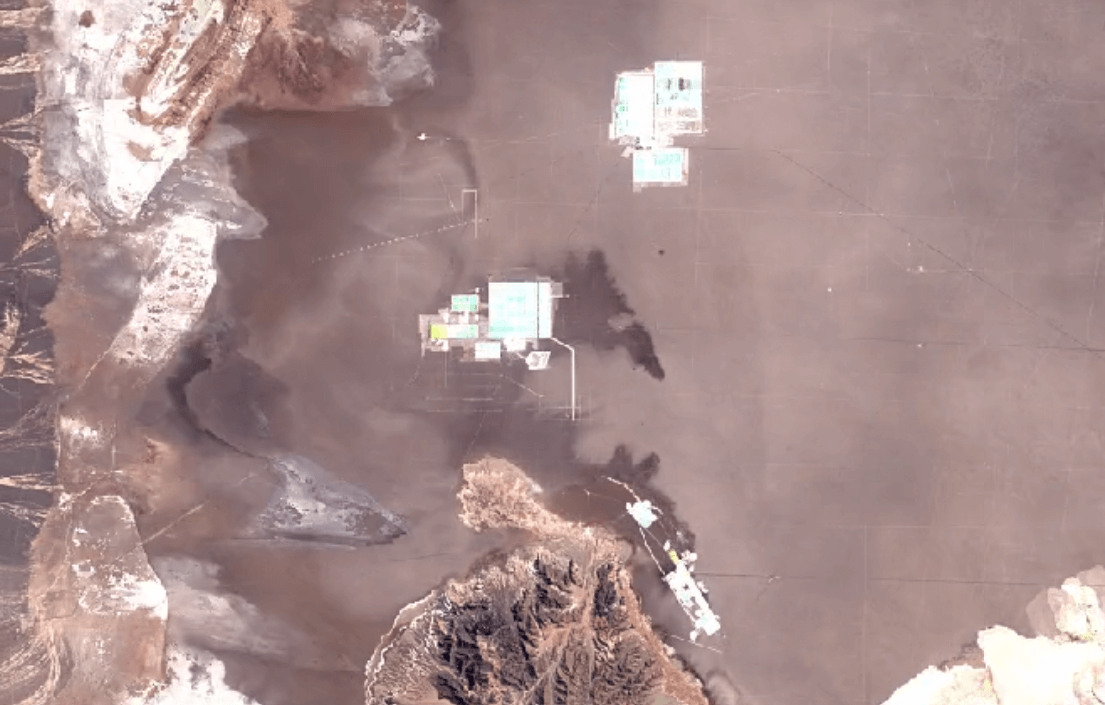

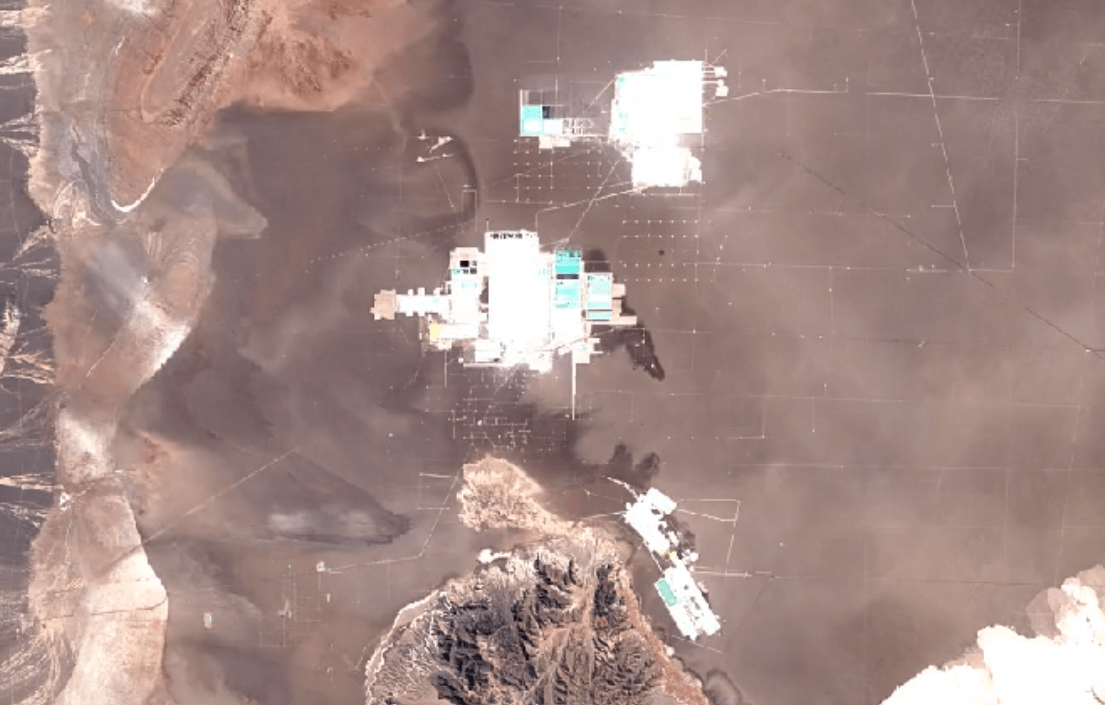
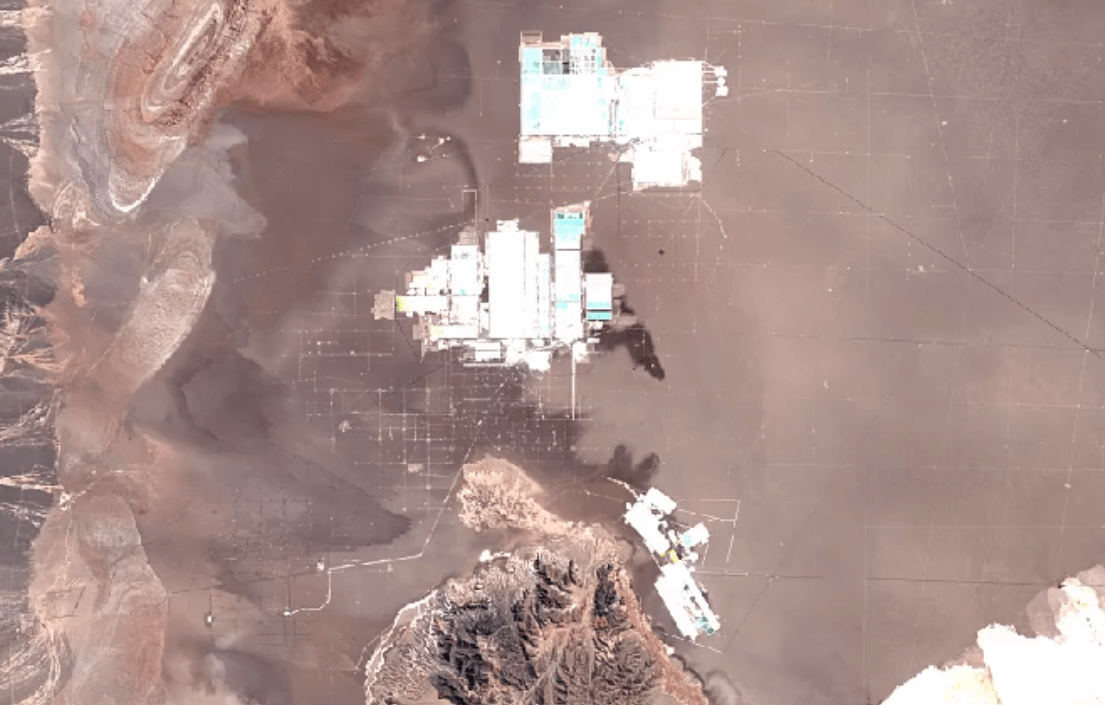
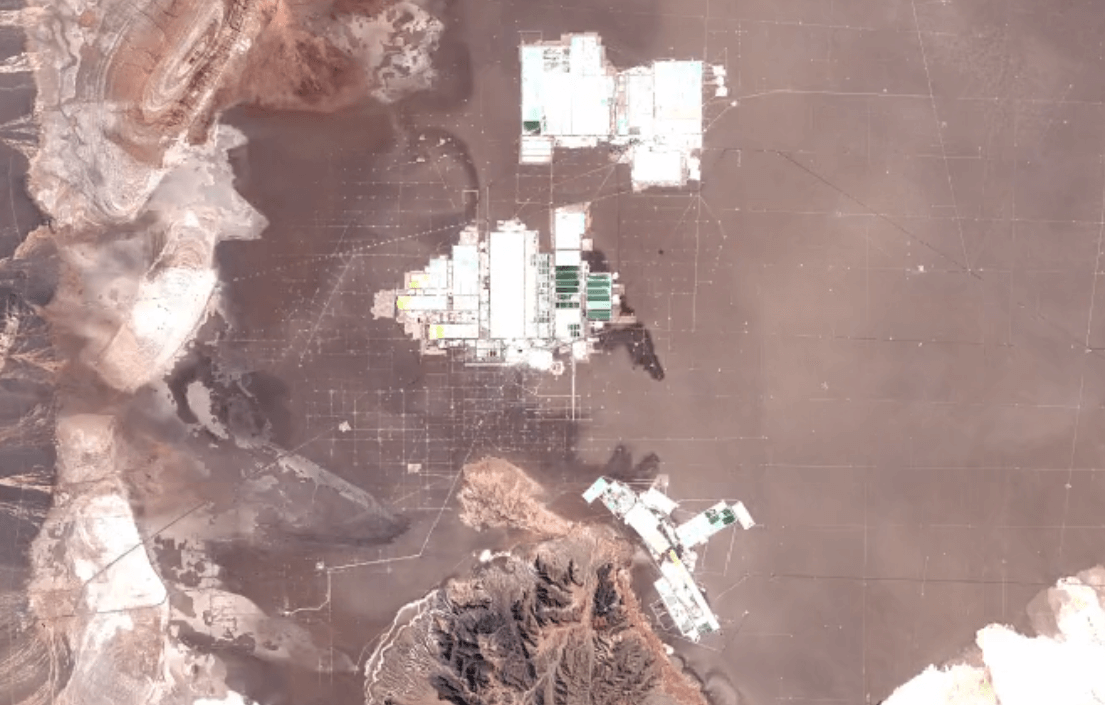
San Pedro de Atacama, ChileImage Attribution: Google Earth Engine
Lithium is primarily mined using brine extraction.
Brine extraction impacts the environment, and impacts people too. Brine extraction drains water from natural underground reserves of drinking water. This water is depleted from the ecosystem more quickly than it can be replaced through the water cycle.
Brine extraction requires toxic chemicals to process lithium. Release of these chemicals harms air, soil, and water quality.
Aluminum
Al

Aluminum is naturally occurring as the mineral bauxite (aluminum oxides and aluminosilicates).
Aluminum metal is very ductile: it can be easily shaped into a thin foil, wire, or thread. Aluminum is also highly conductive, thermally and electrically moving heat energy.
Because of these qualities, aluminum foil is used as the current collector at the cathode of a lithium-ion battery. In the lithium-ion battery of a mobile phone, current collectors take the form of foil and must be conductive enough to receive the electrical current.
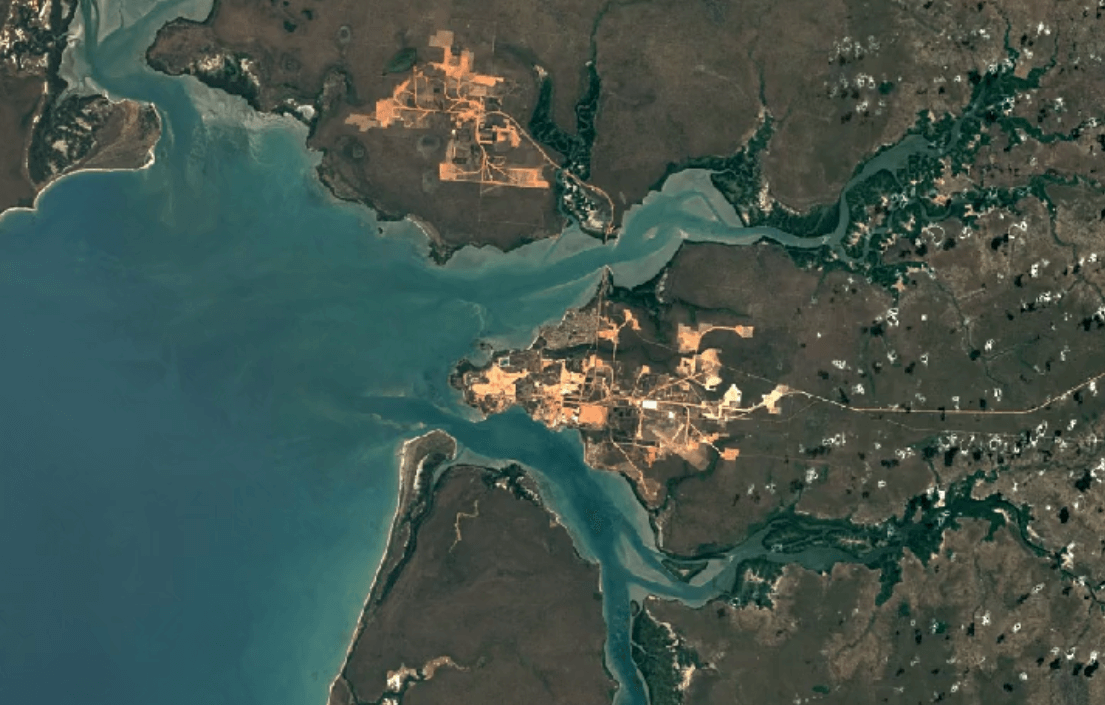
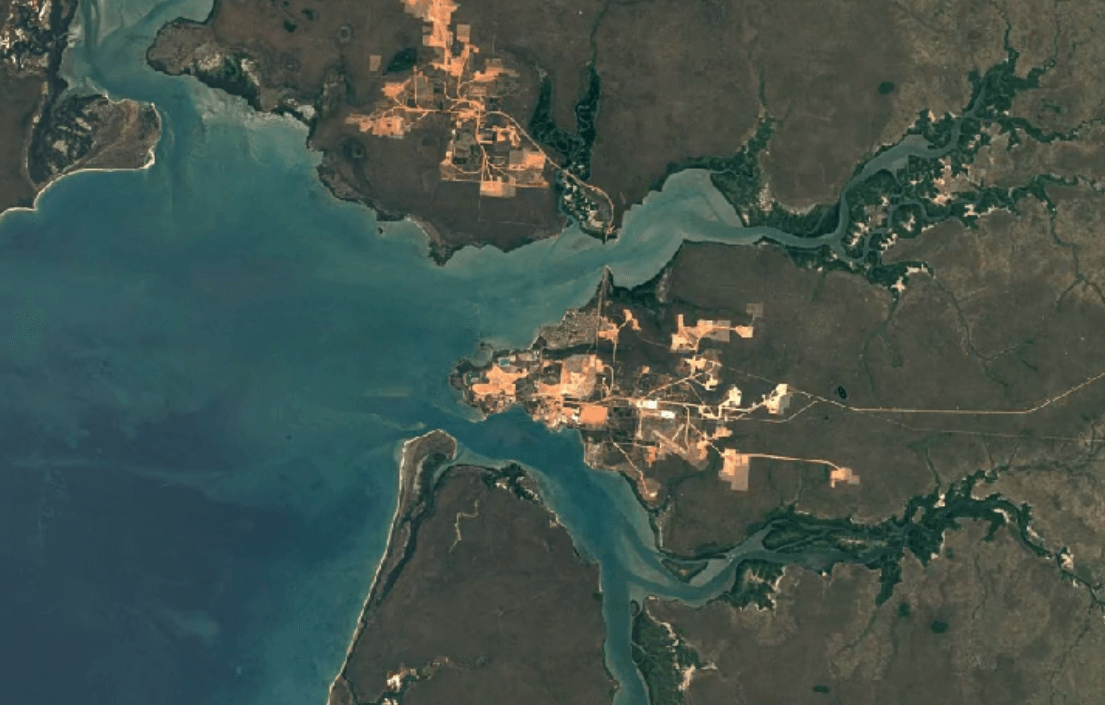
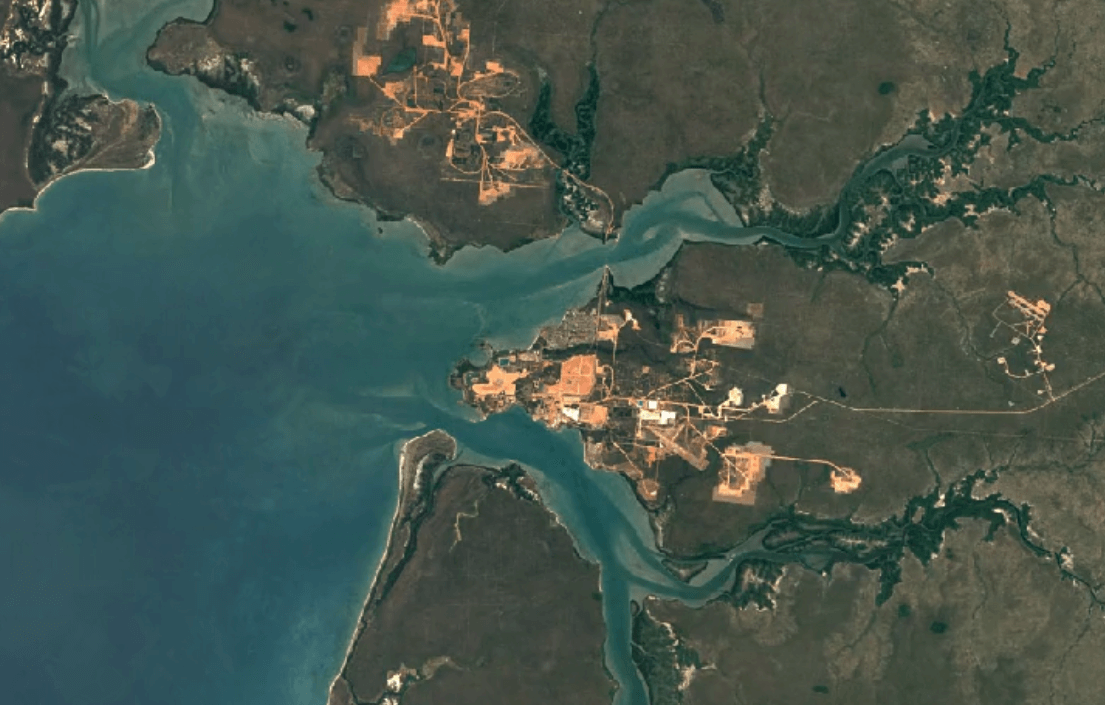
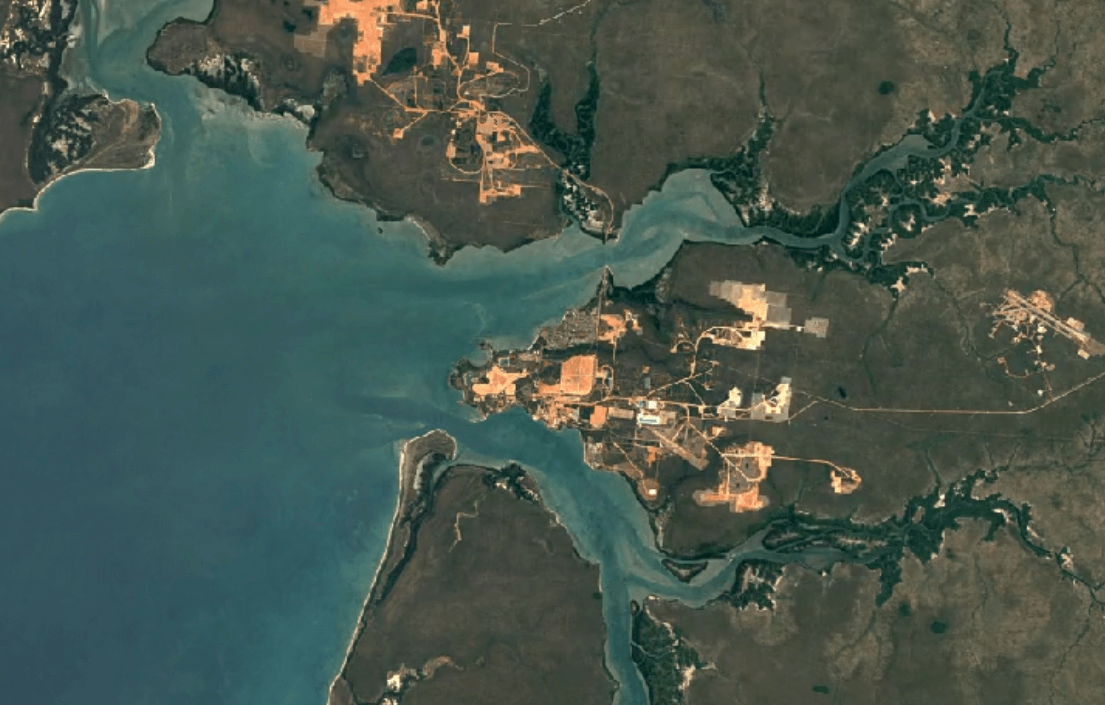

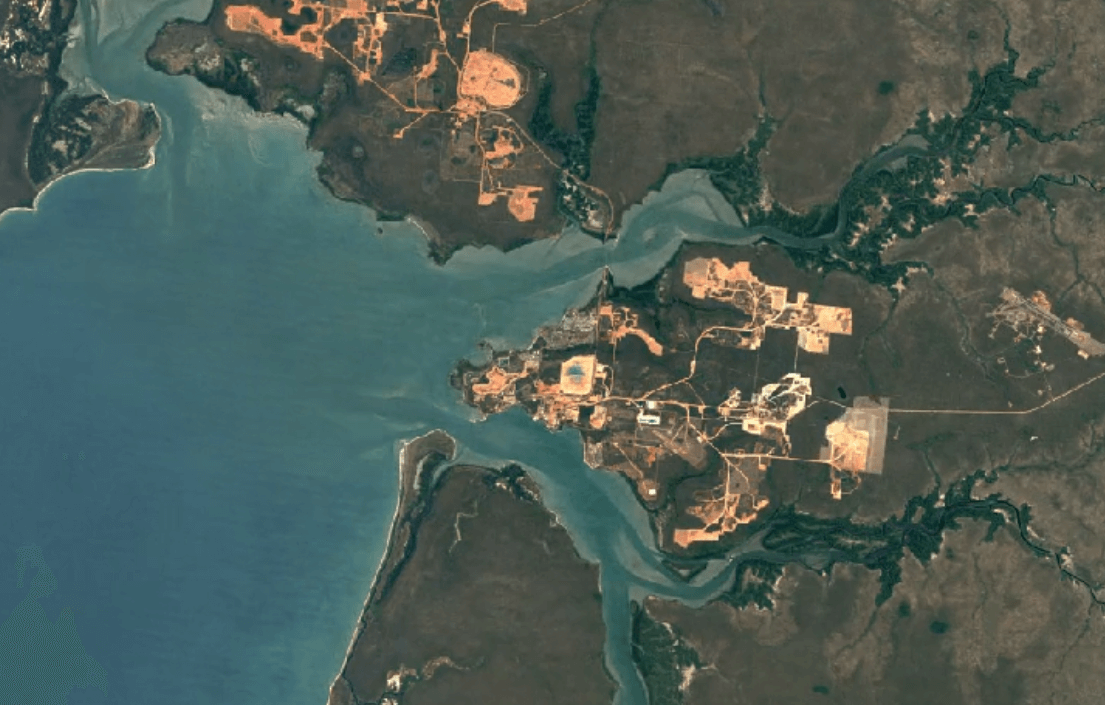

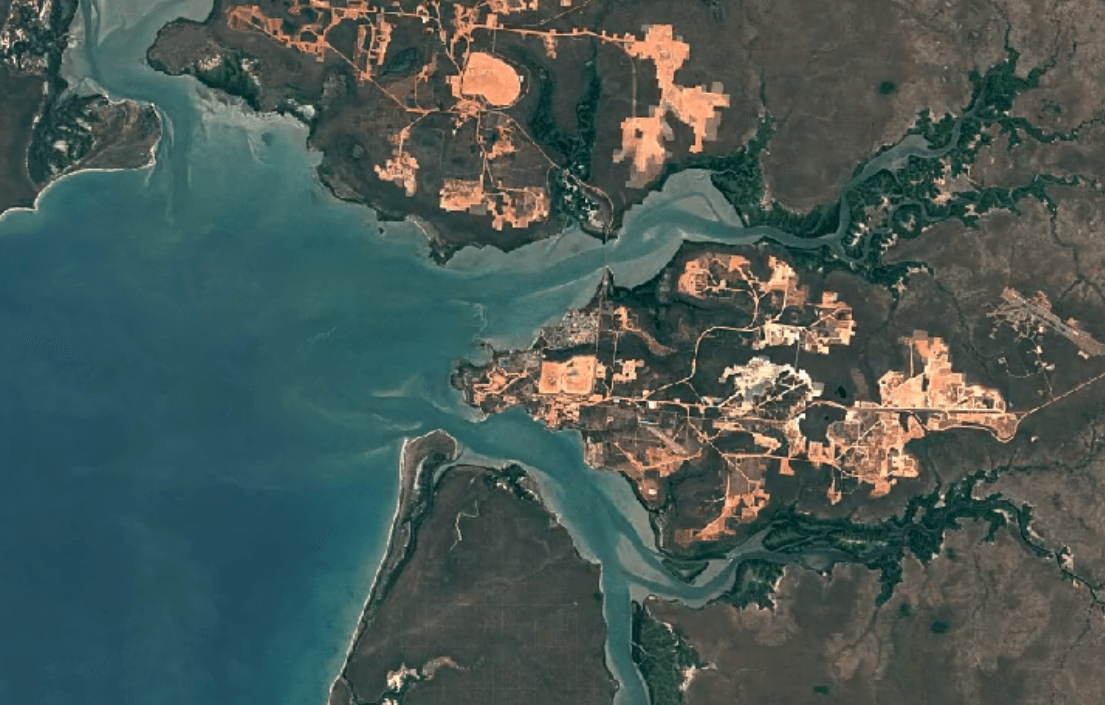

Weipa, AustraliaImage Attribution: Google Earth Engine
Aluminum is primarily mined using open-pit mining.
Human and environmental health risks from mining:
Open-pit mining impacts the environment, and impacts people too. First, in order to make way for an open pit, vegetation must be cleared away. Then, a deep pit is dug. Together, these factors create conditions for erosion.
Mining can create toxic soils and dust with high concentrations of heavy metals like copper and nickel. These dusts are carried by wind into water supplies, crops, and the air. Breathing in, eating food, or drinking water with this contamination puts people and animals at a higher risk of illness.
Carbon (in the form of graphite)
C

Carbon, in the form of graphite, is used as a component of the anode. The porous graphite serves as the location where lithium ions migrate to and from when the battery cell discharges and charges.



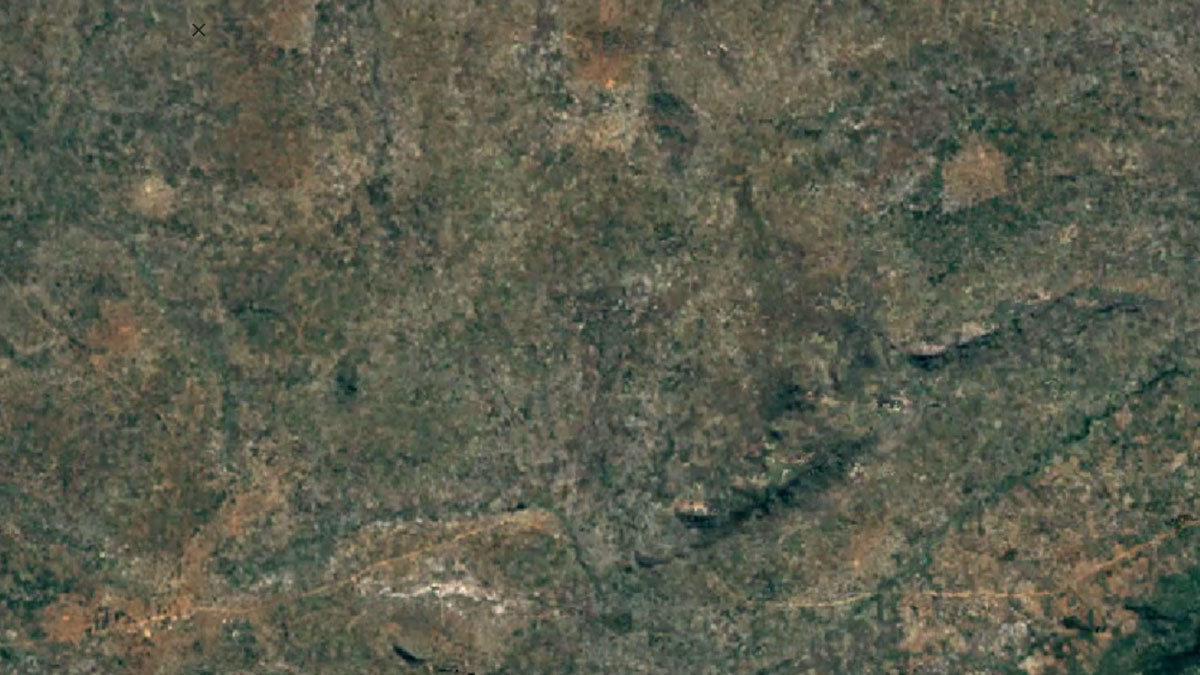
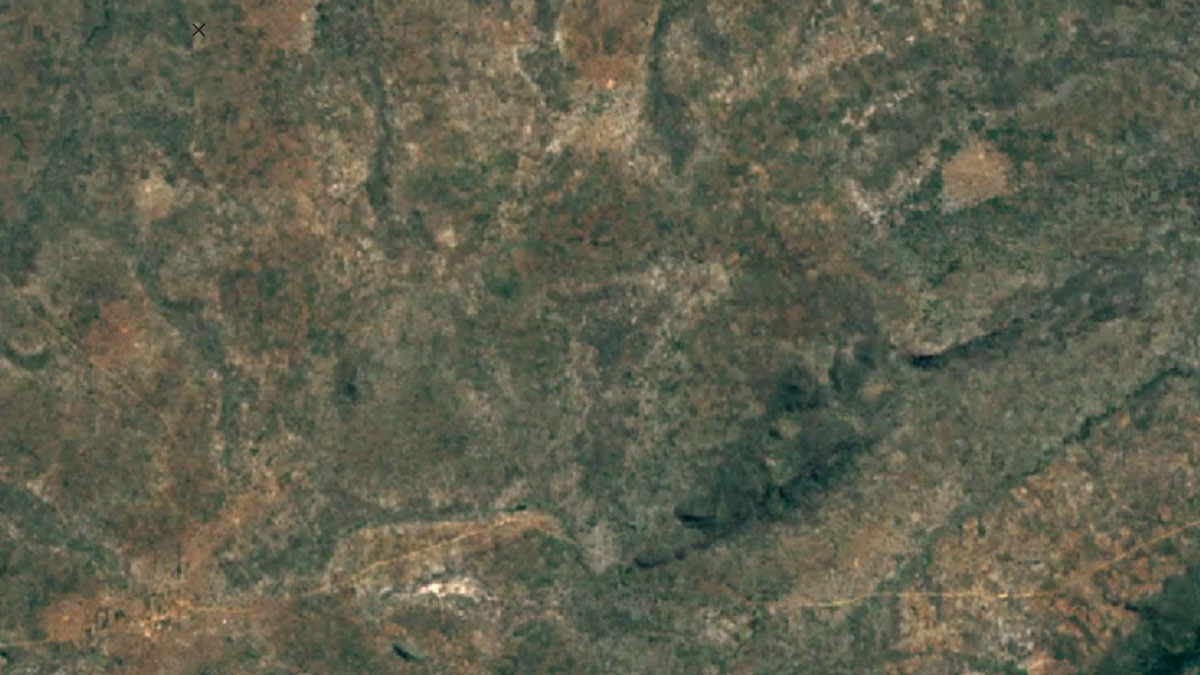
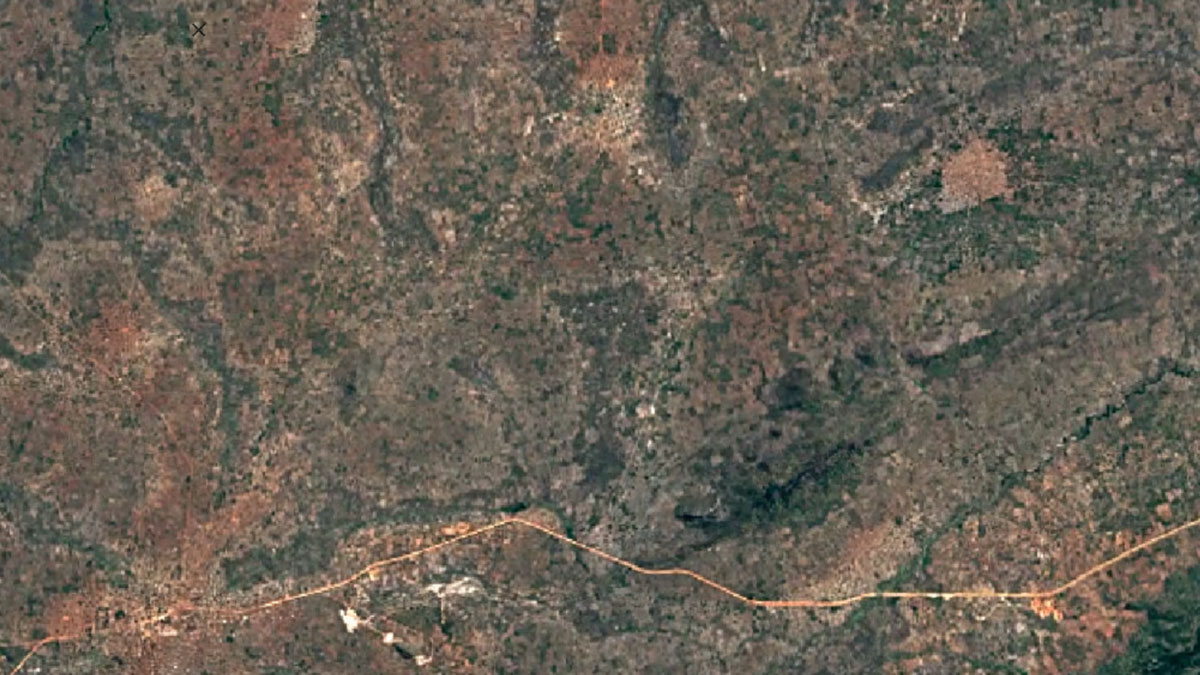
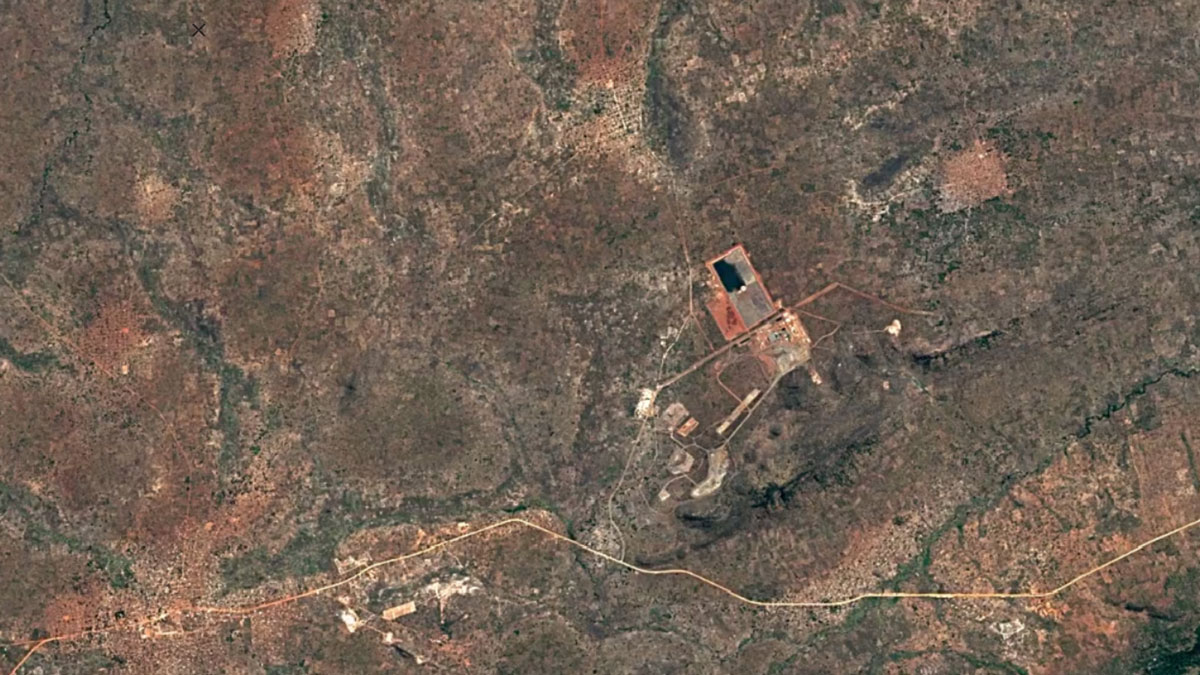
-
Balama, MozambiqueImage Attribution: Google Earth Engine
Natural graphite is mined using open-pit mining, and synthetic graphite is created by refining oil.
Human and environmental health risks from mining:
Open-pit mining impacts the environment, and impacts people too. First, in order to make way for an open pit, vegetation must be cleared away. Then, a deep pit is dug. Together, these factors create conditions for erosion.
Mining can create toxic soils and dust with high concentrations of heavy metals like copper and nickel. These dusts are carried by wind into water supplies, crops, and the air. Breathing in, eating food, or drinking water with this contamination puts people and animals at a higher risk of illness.
Resource extraction is a global undertaking. The kinds of mines you saw above occur all over the world. This map shows where in the world these five main resources come from.
Data courtesy of Mineral Commodity Summaries 2019, U.S. Geological Survey and U.S. Department of the Interior
Resource extraction is a global undertaking. The kinds of mines you saw above occur all over the world. This map shows where in the world these five main resources come from.
Data courtesy of Mineral Commodity Summaries 2019, U.S. Geological Survey and U.S. Department of the Interior
These five minerals (Copper, Carbon (in the form of Graphite), Lithium, Cobalt, and Aluminum) are essential to the functioning of a lithium-ion battery.
Student Reading: Take a Look Inside (PDF 11mbs)
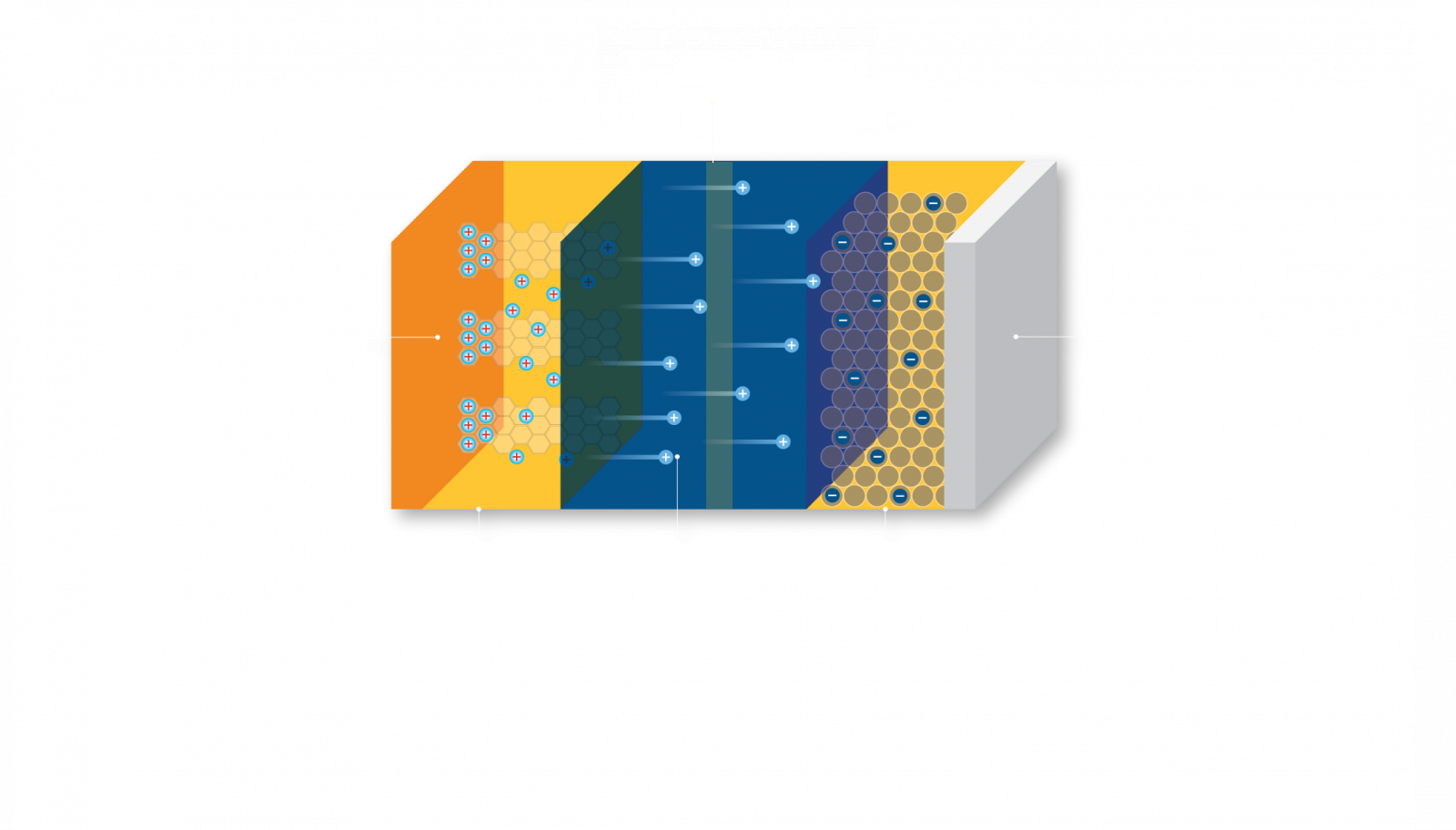
LITHIUM:

Li+ ions move between the battery’s cathode and anode internally, and electrons move in the opposite direction in the external circuit. This migration is the reason the battery powers the device, because it creates the electrical current.
CARBON (IN THE FORM OF GRAPHITE):

Carbon, in the form of graphite, is used as a component of the anode. The porous graphite serves as the location where lithium ions migrate to and from when the battery cell discharges and charges.
COPPER:

Copper foil is used as the current collector at the anode of a lithium-ion battery. In the lithium-ion battery of a mobile phone, current collectors take the form of a foil and must be conductive enough to receive the electrical current.
COBALT:

Cobalt is used as part of an active material (LiCoO2) that is applied to the cathode and acts as a lithium receptor in the electrochemical charge-discharge process.
ALUMINUM:

Aluminum foil is used as the current collector at the cathode of a lithium-ion battery. In the lithium-ion battery of a mobile phone, current collectors take the form of foil and must be conductive enough to receive the electrical current.
