Watching my daughter today run around the house frantically looking for a charger because her battery is low during a facetime call, had me asking her, “What would happen to your tablet if you don’t find your charger?”
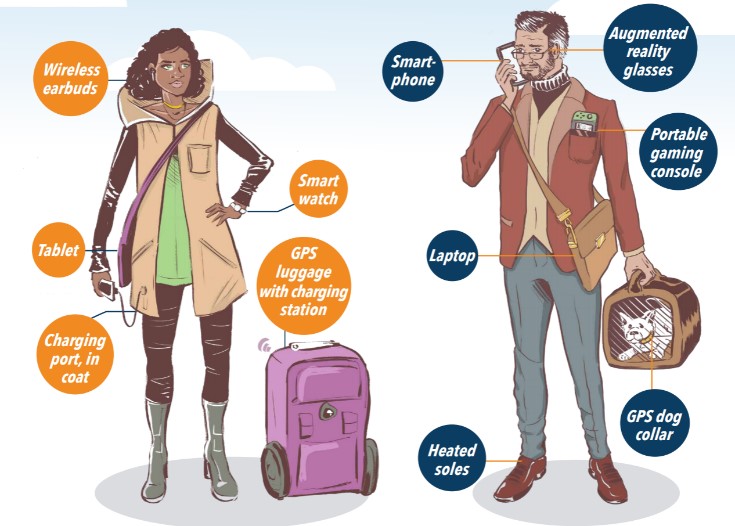 Students today have grown up with smart technology, such as smartphones, smart homes, and even smart wearables. As advanced as this technology is from that of even two decades ago, the same limitation still exists – the lithium-ion battery. While chips and components are becoming more efficient, they become useless if the battery isn’t charged. Recharging phones and tablets have become part of a daily routine for most students, but only after attempting to squeeze every last trickle of power watching one more viral video, clicking to the next Netflix episode, or sharing a video of a cat lip-syncing to the latest trending soundtrack.
Students today have grown up with smart technology, such as smartphones, smart homes, and even smart wearables. As advanced as this technology is from that of even two decades ago, the same limitation still exists – the lithium-ion battery. While chips and components are becoming more efficient, they become useless if the battery isn’t charged. Recharging phones and tablets have become part of a daily routine for most students, but only after attempting to squeeze every last trickle of power watching one more viral video, clicking to the next Netflix episode, or sharing a video of a cat lip-syncing to the latest trending soundtrack.
While 99.9% of all students know exactly where a charger or charging station is located at all times, (number reported is for facetious attempts only…kind of) many never stop to think how dependent they have become on lithium-ion batteries. I believe most students would not be able to tell me where lithium-ion batteries come from, what would happen if a link was broken in the battery supply chain, and where do all the old batteries go?
Hello, can you say “teachable moment!” Not only can you tie this subject into all disciplines of science, whether you’re teaching life, earth, or physical science, but the subject matter is so relevant – not only in regards to innovative technology but also to solving real-world problems of e-waste and thermal runaway.
*screech* My teacher brain just threw the brakes on. I knew I needed to find a lesson that could provide students with the understanding of the questions I was about to propose, but also knew this lesson had to work whether the school year offered in-classroom situations or distance learning situations due to the ongoing pandemic. Guess what? I found exactly what I was looking for – a free, national standards-aligned middle school science lesson, designed to be used independently or during full class instruction created by UL Xplorlabs: Extraction to E-Waste: The Lithium-Ion Battery Supply Chain.
This latest module uses interactive videos, relevant articles, instructional experiences, and creative challenges to provide students information to answer these essential questions:
- What are the trade-offs we make as we become more dependent on lithium-ion batteries?
- What is a supply chain? What are the links along the supply chain of lithium-ion batteries?
- What are the solutions to the growing epidemic of e-waste?
The Extraction to E-waste lesson supports student understandings of the Disciplinary Core Ideas (DCI) from the United States’ NGSS Middle School Physical, Life, and Earth Science, therefore it compliments my current curriculum.
Physical Science
- MS-PS1-3: Gather and make sense of information to describe that synthetic materials come from natural resources and impact society.
Life Science
- MS-LS2-4: Construct an argument supported by empirical evidence that changes to physical or biological components of an ecosystem affect populations
- MS-LS2-5: Evaluate competing design solutions for maintaining biodiversity and ecosystem services.
Earth Science
- MS-ESS3-3: Apply scientific principles to design a method for monitoring and minimizing a human impact on the environment.
- MS-ESS3-4: Construct an argument supported by evidence about how increases in human population and per capita consumption of natural resources impact Earth’s systems.
Engineering Design
- MS-ETS1-1: Define the criteria and constraints of a design problem with sufficient precision to ensure a successful solution, taking into account relevant scientific principles and potential impacts on people and the natural environment that may limit possible solutions.
- MS-ETS1-2: Evaluate competing design solutions using a systematic process to determine how well they meet the criteria and constraints of the problem.
 What I love about this Extraction to E-waste module is that it doesn’t assume what students already know and begins with the basics, such as how a lithium-ion battery works and the components of a lithium-ion battery. This not only gives students an adequate background needed to successfully understand the module, but more importantly, it reinforces what they have learned in their science units. For example, from a knowledge stand-point, students have learned about elements and their properties, and now in this module, they are able to connect an element and its use to an active material and compound, such as cobalt pictured here.
What I love about this Extraction to E-waste module is that it doesn’t assume what students already know and begins with the basics, such as how a lithium-ion battery works and the components of a lithium-ion battery. This not only gives students an adequate background needed to successfully understand the module, but more importantly, it reinforces what they have learned in their science units. For example, from a knowledge stand-point, students have learned about elements and their properties, and now in this module, they are able to connect an element and its use to an active material and compound, such as cobalt pictured here.
From an engineering design stand-point, students learn about a real-world application of the design process in which students must first understand the trade-offs behind both the problem and its potential solutions. Students use their knowledge of these trade-offs in order to inform their design: What must change? What are their priorities and constraints?
 Throughout the module, students will explore relevant articles and videos that will provide them with the understanding of supply chains, lithium-ion batteries and the phenomenon of thermal runaway, and the problems created by e-waste so they can aspire to the solutions being posed and begin to innovate their own idea.
Throughout the module, students will explore relevant articles and videos that will provide them with the understanding of supply chains, lithium-ion batteries and the phenomenon of thermal runaway, and the problems created by e-waste so they can aspire to the solutions being posed and begin to innovate their own idea.
With so much information offered, I want to break it down so students become “experts” in one part of the battery supply chain, so they can share their knowledge to their classmates.
Each student will be giving the student reading, On The Mark, a magazine which publishes content from various authors and explores the intersection of safety, science, and technology and is provided in the module.
Students will then be grouped and assigned to one of the three steps of the battery supply chain:
- Batteries and Safe Cities
- Explore the Issues
- Explore Solutions
While students are researching, I can easily check for understanding using prompts for student discussions offered in the Teacher Guide. These discussions can easily be extended into further investigations.
I believe after this completing this module, not only will students have a much better idea of lithium-ion technology and its supply chain, but will now consider their benefits and harms when it comes to our lives and communities. These reflections will continue onto further experimentation, and allow these students to be the future of technology design and engineering solutions for years to come.